

New eCOUCH project develops route towards efficient electrochemical CO2 utilisation
03-06-2019 | New Project | P2Chemicals
 VoltaChem/TNO, Avantium and the University of Amsterdam are joining forces in the development of a highly efficient electrochemical process for the conversion of carbon dioxide. In the eCOUCH project, they aim to demonstrate at the bench scale how paired electrolysis can produce both chlorine and carbon monoxide, starting from hydrochloride acid and carbon dioxide. The high electron efficiency of the process promises a much better business case than more conventional electrochemical CO2 conversion approaches
VoltaChem/TNO, Avantium and the University of Amsterdam are joining forces in the development of a highly efficient electrochemical process for the conversion of carbon dioxide. In the eCOUCH project, they aim to demonstrate at the bench scale how paired electrolysis can produce both chlorine and carbon monoxide, starting from hydrochloride acid and carbon dioxide. The high electron efficiency of the process promises a much better business case than more conventional electrochemical CO2 conversion approaches
The eCOUCH project (an acronym for 'electrochemical CO2 Utilisation with Chlorine'), partly funded by the Dutch Enterprise Agency RVO, addresses the major societal challenges of cost- and energy efficient utilization of CO2 on the one hand, and the storage of renewable energy on the other. It aligns with VoltaChem's Power-2-Chemicals program line aimed at the development of electrochemical routes that produce industrially relevant chemicals driven by renewable electricity. Storing renewable energy in such 'platform molecules' will be critical for a future European sustainable energy and industry infrastructure.
Highly efficient paired electrolysis
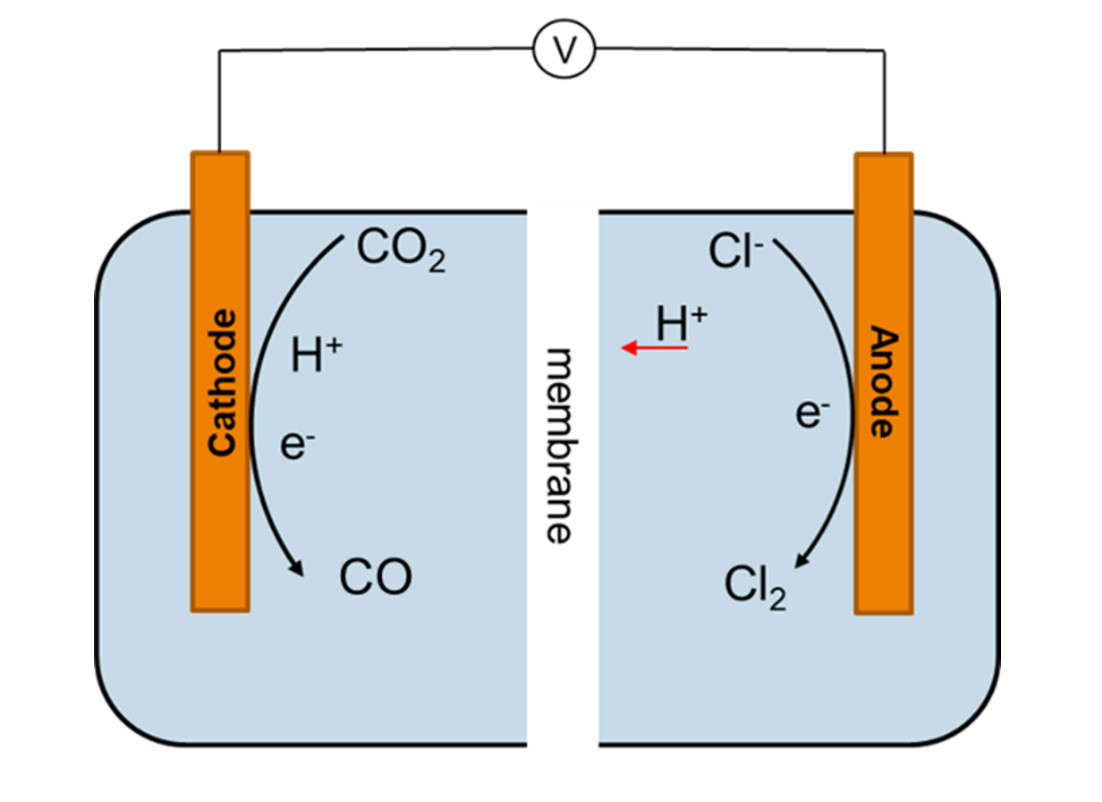
Central to the eCOUCH project is the concept of paired electrolysis. Most often in electrolysis, the focus is on just one of the two electrodes as the driver for the desired conversion. The chemistry that occurs at the counter electrode then yields a product that holds little economic value. The paired electrolysis concept implies the synthesis of valuable products at both electrodes. The starting point of eCOUCH is the so-called chloralkali process. This is used on a large (multiple megawatts) scales to produce chlorine (Cl2), and yields hydrogen (H2) and sodium hydroxide (NaOH) at the counter electrode. Although hydrogen in itself has some value, the eCOUCH team aims to complement this counter process with the conversion of CO2 to CO. A scheme of this process is showed in the feature visual above.
As a result, valuable chemicals will be produced in a highly efficient process. Since useful chemistry occurs at both electrodes, theoretically an electron efficiency of up to 200% can be achieved. The paired electrolysis will thus reduce the capital costs per product up to 50%. Combined with the generation of H2 at the same cathode, the electrolysis cell will yield the so-called syngas (a mixture of CO and H2). This can be used as a feedstock for the synthesis of hydrocarbons (in a Fischer Tropsch process).
Strong drivers for application
The eCOUCH project will thus develop breakthrough technology for the use of renewable energy for the conversion of waste materials (HCl and CO2) to valuable platform chemicals CO and Cl2. Carbon monoxide is an industrial gas that has many applications in bulk chemicals manufacturing. Cl2 is widely used for the synthesis of chlorocarbon based intermediates. The proposed process could be of value at many chemical sites where both CO2 and HCl are already available.
The project partners expect the use of paired electrolysis to enhance the viability of the business case. What’s more, compared to other means of converting CO2, the electrochemical approach has the advantage of high efficiencies, ambient conditions, and high selectivity towards CO. Another strong driver for application is that the proposed paired electrolysis could also lower the carbon footprint of the chloralkali process.
Better catalyst, better electrodes, optimal design
The first experimental explorations of the concept have shown that some crucial problems need to be solved. One of these is that the selectivity towards CO rapidly deteriorates, which requires research in catalyst development and electrode-design. This will be mainly the responsibility of Dr. Ning Yan of the University of Amsterdam, working at the Research Priority Area Sustainable Chemistry and the Van 't Hoff Institute for Molecular Sciences. He will focus, amongst others, on the use of metallic nanoparticles as catalysts.

Scientists of the eCouch project. From left to right: Dr. Ning Yan (UvA), Dr. Klaas Jan Schouten (Avantium) & Dr. Roman Latsuzbaia (VoltaChem/TNO)
Dr. Klaas Jan Schouten, program manager electrochemistry at Avantium, will focus on the industrial perspective, and will also bring in the company's expertise in gas-diffusion electrodes. An optimal electrode design will promote the reaction rate of the electrochemical CO2 reduction. Finally, Research Scientist Dr. Roman Latsuzbaia of TNO's Sustainable Process & Energy Systems group will focus on the engineering and production of the bench scale demonstration reactor, and prepare towards scale-up.
Scale-up towards industrial scale
The ultimate goal of the ECouch project is to develop electrolyzers of at least the scale that is currently the standard in chloralkali processes, both as stand-alone and as a retrofit option. This is why the current partners are already in discussion with several interested chemical companies in the polyurethane and polycarbonate polymer value chains to further develop the technology to fit in their business. It is expected that this will lead to several follow-up projects in the coming years
Are you interested in Power-2-Chemicals technology?
Contact our Business Development Manager Willem Frens to discuss how VoltaChem can be of benefit to you.
Share this page: